|
Carbohydrates
Glucose—the most universal sugar:
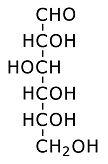
Here's the three dimensional ring structure of glucose: Glucose: 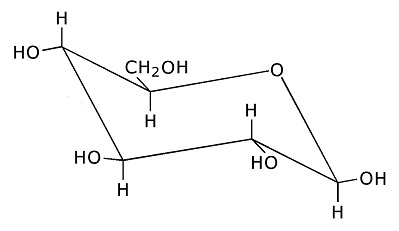
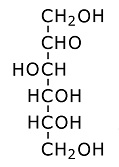
Glucose forms a six member ring. Oxygen is one of the ring members, as usual with sugars, while one of the carbons is attached to the side. (Each branch point in the drawing is a carbon atom.) Each of the carbons has an OH group attached, which is usually the case for sugars. The OH is called a hydroxyl group. It is somewhat polarized toward negativity. This stems from the tendency of oxygen to pull electrons toward itself. The negative polarity of the hydroxyl groups on sugars increases solubility in water, because water is highly polarized and is therefore drawn to other polarized molecules. (Water is an oxygen atom with two hydrogens covalently bound to it.) The hydroxyl groups on sugars also facilitate metabolism, because they are broken down more easily, and yield energy more easily, than hydrogen attached to carbon. Polysacharrides:Saccharide is a scientific way of saying sugar. Poly means several linked together. Polyglucose can take the form of starch or cellulose. The difference is in the branching. More cross branching between chains gives cellulose more strength. Often sugars combine in pairs. The pair is then called a disaccharide. An example is sucrose, which is common table sugar. It consists of glucose and fructose linked together. The structure of fructose is this: Fructose: 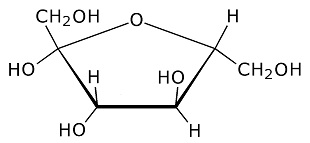
Fructose is a six carbon sugar which forms a five member ring. Oxygen is one of the ring members. When it combines with glucose to form sucrose, a hydroxyl oxygen is shared with glucose creating the link.
|