Chapter 2: The Morel Mushroom—Extreme Evolution Scientists Got Wrong
Chapter Summary The biology of the morel mushroom is not really studied by university scientists, because broad subjects are too complex for the narrow focus at universities. The morel evolved from a yeast growing at the base of trees and extending into the soil where rich bacterial nutrients exist. The resulting mushroom is not well adapted, and it dies out in about two ice age cycles (200 thousand years). It re-evolves during each ice age cycle. Other scientists claim it is 129 million years old (1). Such extreme errors should not be possible where there is real science. Phylogenetics is part of the problem. It is a modern way of scrambling taxonomy using trash computer programs applied to the study of molecules. It's like buying a house by looking at one square inch of its surface. Elaborate studies of DNA can provide useful information on evolution, but phylogenetics is way over-simplified. ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ ◆ The morel mushroom (genus Morchella) evolved recently from a yeast which formed a mushroom about 20 thousand years ago. Other morel scientists claim it is an ancient cup fungus 129 million years old. The mushroom hasn't formed square corners on the cells, and it can't measure gravity to determine vertical direction. Everything which emerges from the ground measures gravity to grow vertical, but the morel hasn't gotten that far.
Such an extreme divergence from reality occurred because university scientists do not study whole organisms; they study laboratory procedures. To understand mushrooms, you have to study a lot of ecology and evolution and spend a lot of time outdoors looking. In fact, you probably need an agriculture background, because only agys study the soil. Biologists don't study soil. Even if they study soil ecology, they only look at the organisms they are concerned with, and they don't understand soil. I studied agriculture in high school in South Dakota, and I started college in agriculture. I later switched majors to microbiology.
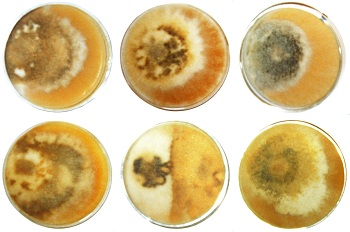
Morel scientists never noticed that morels only grow in sandy soil or something similar such as mountain humus. Morels follow sandy river basins and become quite isolated due to difficulty getting ascospores out, which results in a lot of localized variations. Sand has the characteristic of not drying out, because it lacks capillary action for moving water to the surface. The morel does not tolerate drying, because yeasts don't. Yeasts evolved from filamentous fungi after modern biology began 65 million years ago. They adapted to sugary solutions produced by modern plants. Fossil evidence shows yeast going back only 50 million years (2). The significance of these points shows up in the erroneous claim that a leaf mold (Costantinella cristata) is a conidial stage of the morel. Conidia are microscopic stalks with exposed spores on them—the most common way for molds to form spores. Early in the last century, Molliard attempted to grow morels on apple compost in the ground. He inoculated it with morel tissue and covered it with leaves. A white sheet of leaf mold covered the surface before a few morels came up. So he studied the leaf mold claiming it was a conidial stage of Morchella. The assumption was totally unwarranted, but it still persists. The leaf mold was said to be found in great abundance on dead leaves in the forest. Morchella could not grow in that manner, because it does not tolerate exposure without dehydrating. Also, it is not a decay organism, as it would have to be to grow competitively on dead leaves. Morphological complexities were also uncharacteristic of Morchella including microstructures such as crosiers and rosettes in addition to conidia. Microstructures take millions of years to develop. That type of evolution occurred between 100 and 300 million years ago and should not be expected to be occurring at this time. There are windows of opportunity for evolution based on stages of complexity and competition. The window closes for the type of evolution that occurred under more primitive conditions. In graduate school, I studied yeast physiology. The yeast that I studied, Nadsonia fulvescens, had the same physiology as mushrooms. This physiology would have been impossible to study directly in mushrooms. The physiology of filamentous fungi is almost unstudied, because the organisms cannot be grown in liquid media as yeasts and bacteria can. Microbial physiology is very highly developed because of the ease of using liquid media and the speed of growth. The study of physiology in higher organisms is largely built upon the work done with microbes. It's a lot easier to study physiological effects after the basics have been established. There are two major physiological patterns that I found in the yeast and they apply to mushrooms. One is endotrophism and the other is energy (ATP) control over differentiation. Differentiation means formation of a new structure such as a spore or mushroom. Endotrophism means nutrition from within. It showed up as the formation of spores in distilled water. This was surprising, as yeasts were assumed to require nutrients while spores formed. In retrospect, it can be said that this yeast is the only one which produces spores endotrophically, because there is a telltale sign. Cell material must migrate into a smaller chamber. There is a significant reduction in cell mass, as energy gets burned up and new material is synthesized. So the entire cytoplasmic mass migrates into a smaller chamber where the spore forms. The new chamber is about one third the size of the original cell. No other yeast shows this migration of cell material into a chamber where the spore forms. There are, however, undiscovered yeasts in the wild. Nadsonia was forced into endotrophism, because it adapted to growing on tree sap on the surface of trees. When rain would wash the yeast cells away from their nutrients, they would die off, unless they formed a spore. The other major physiological process was the control of differentiation through a peak in the ATP level. This mechanism was first suggested by A.F. Croes in 1968. Yeast scientists knew there was a trigger mechanism with sporulation, but they could not determine what caused it. They tested every chemical on the shelf to see if it would trigger sporulation and found nothing. Croes looked at energy metabolism and found that it reached a peak just when sporulation was triggered indicating a causative effect. I found additional evidence in nitrogen depletion. Without nitrogen, synthesis of cell material stops, while energy metabolism continues. This results in an accumulation of ATP, because much is being produced and little is being used. Sporulation was enhanced under these conditions. Mushrooms show the same physiology in the way the composting procedure works. First, mycelium is grown on compost containing straw which is attacked with excreted enzymes. After about a month, the mycelial mass gets large, and it is covered with a layer of peat moss called a casing. After another month or two, the mycelium gets to the surface of the casing, and a mushroom forms. Afterwards, the mycelium has nearly disappeared, as the cell material was transferred upward into the mushroom. The transfer of cell material is an endotrophic process. Soil type mushrooms must be endotrophic to speed up formation of the above ground structure, because the tissue dries rapidly. It must get spores formed and into the air within two or three days. An exception is the bolete (Boletus edulis, porcini). It's tissue does not dry out, so it takes a month or more to gain full size, and it does not need to store up a large mass in the mycelium first. The bolete evolved a high degree of sophistication over hundreds of millions of years. Only the puffball is older. Their tissue is virtually the same. It's like tough marshmallows, and it evolved away all flavor as a means of preventing animals from eating it. The spore area of the bolete has much flavor; and later, the rest of the tissue acquires much flavor to encourage animals to eat it and carry spores around. With the composting procedure, the mushroom does not form until the mycelium gets to the surface of the casing. The only difference between the surface and within the casing is oxygen availability. The only thing oxygen does is create respiration for the synthesis of ATP. This means a peak in the ATP level induces mushroom formation. This effect occurs with all fungi and is usually visible as spores forming on the surface. The ATP level is the universal signal for conditions suitable for differentiation with fungi. It says cell machinery and nutrition are adequate to complete the process. I studied the morel because of its extremely mysterious nature. Why would a mushroom produce ascospores within the tissue, which is extremely problematic and limits the ability to get spores out? The answer was found when growing mycelium in jars of sterile peat moss with various nutrients. With two jars sitting along side each other, one had thick, white mycelium, while the other had little. Checking the make-up of the cultures, the only difference was that one of them contained glucose, and the other one did not. The one containing glucose had little mycelium, while the one without glucose had much mycelium. Glucose is an ideal nutrient for all living organisms except photosynthesizing plants. If a nonphotosynthesizing organism doesn't get glucose in its diet, it will synthesize it, because it is needed as a starting point for some synthesis reactions. The only reason why glucose could have inhibited mycelium growth would have been the production of acid as a byproduct; and only yeasts excrete acid as a byproduct of glucose metabolism. It meant the morel had the same metabolism as yeasts. Morel mycelium thrives on glucose, but under these conditions, acid produced from glucose was allowed to accumulate to toxic levels. Filamentous fungi excrete nothing but carbon dioxide under most conditions. The reason is because anything which is excreted would accumulate on the mycelium and damage it or kill it. This means the morel didn't evolve the excretion of acid for some purpose; it had to recently acquire the property from its ancestor, which had to be a yeast. The morel continues to excrete acid as a method of feeding on bacteria in the soil. The primary bacterium which it feeds on would be Pseudomonas fluorescens. This bacterium flourishes in wet, spring soil, which morels grow in. The bacterium has a strong symbiotic relationship to plants. Evolutionary age increases symbiosis. The pseudomonad has had plenty of time to adapt, as it is approximately the oldest living creature on the planet. Blue greens are an exception, as they evolved into a dead end in polluted water. Pseudomonas fluorescens (P.f.) had similar characteristics to its present ones including two polar flagella for rapid motion in water about a billion years ago. About 700 million years ago, it evolved the modern mechanism for ATP synthesis for respiration from its rotating flagella. When terrestrial life began during the "Cambrian explosion of life" 543 million years ago, P.f. would have been the first bacterium to enter the soil. It is now the best adapted soil bacterium and the best adapted water bacterium. It has versatility and sophistication incomparable to any other bacterium. The way it feeds plants is by breaking down proteins and other biological materials in early spring soil and then self-destructs (called autolysis) to release more refined nutrients into the soil for itself and other species. All bacteria and yeasts undergo autolysis as they age or die off as a method of recycling nutrients. They break down large molecules into their subunits including amino acids and nucleic acids. Plants then get these pre-constructed molecules as ideal nutrients. The morel has become dependent upon such nutrients, though it will grow on more rudimentary nutrients including glucose and ammonium sulfate. The morel can easily induce autolysis in P.f. by excreting acid, as the bacterium will break down at pH 5.0 and lower. This pH is surprisingly high for bacterium, resulting from its highly developed symbioses with plants. Plant roots excrete small amounts of acid as nutrients to promote bacterial growth around them. The roots can then excrete more acid to induce autolysis in P.f. to feed on it. This situation was ready to be exploited by the morel mushroom due to the natural tendency of yeasts to excrete acid. The yeast which the morel evolved from appears to be Schizosaccharomyces japonicus, as they both have eight spores lined up in a row in the ascus. The yeast grew at the base of trees and extended mycelium into the soil to feed on bacteria. This is indicated by a high tendency of morel mycelium to revert to a pattern of surface growth under laboratory conditions. The surface growth forms a tissue with a degree of differentiation including the creation of pigments with some patterns similar to a mushroom surface. This growth is highly anomalous and very informative. The anomaly scrambles the characteristics of the morel mixing recent forms with earlier ones. It creates a rubbery tissue on the bottom, which sits on a hard gel called agar with nutrients. The cells on top become elongated and some filamentous. The elongated cells often break into arthrospores, which is an adaptation to water by the morel. The tissue cells are rounded, like potatoes, without square corners. The morel has not formed square corners on the cells, because microstructure is much more difficult to evolve than macrostructure. Cells must be very highly ordered to maximize efficiency. A little sacrificing of efficiency won't work, because there is too much complexity. The reactants move from enzyme to enzyme with a minimum of space between each one to prevent reactants from floating around and getting in the way of each other. The enzymes are held in place through attachment to membranes. It's no easy thing to re-order the arrangement. Evolution of microstructure requires a re-ordering. So the microstructure of the morel is still very much like the yeast which it evolved from. Twenty thousand years of evolution is nothing for changing microstructure. The microstructures of filamentous fungi mostly evolved between two hundred and three hundred million years ago. Evolution was much easier early on than it is now, because there was less specialization and interdependence. There is so much specialization now that all biology is on the verge of becoming extinct due to too much interdependence between functions. The large number of species becoming extinct at this time is greatly influenced by the over-specialization which makes adaptation difficult. There are remnants of microstructures in filamentous fungi (molds) which were extremely frivolous due to the ease of evolution and little for competition creating demands for efficiency. Variations of the clamp connection show this. A clamp connection is a tube that grows out the side of a filament and circles around a cross wall to re-enter the adjoining cell. Sometimes nuclei would pass through the clamp connection, and sometimes gene exchange would occur within them. To some extent, ancient structures are not totally abandoned by evolution when no longer needed. There must be a selective advantage in producing change before it will occur. Without advantage for change, remnants of structures will be carried for hundreds of millions of years. An example of this is two small dots on a bone below the front teeth of humans. Those dots can be felt with the tongue. They are remnants of hooks used by ancient fish to catch prey. They evolved down to a point of irrelevance and were then maintained religiously in the DNA without alteration for about four hundred million years. This shows that evolution discards nothing in the DNA. This may be part of the reason why ninety percent of human DNA does not appear to have a function. Some of it has invisible functions, such as coding for regulatory RNA, as showing up in recent molecular biology. Eight percent of human DNA is old retrovirus DNA. Some of it gets adapted to new purposes. The morel shows that patterns of physiology are also carried through evolution without being discarded. The morel anomaly reverts to patterns of growth on a surface which were once needed but no longer have a use in nature. It also shows a scrambling of characteristics, where recent pigments are mixed with irrelevant tissue. The morel also continues with disadvantageous characteristics which will eventually evolve away but haven't had enough time yet. An example is residual autolysis, which is breakdown of tissue. The yeast ancestor used autolysis to recycle nutrients. The morel gains nothing from autolysis. It causes mycelium to deteriorate rapidly in a laboratory, and it causes the tissue to break down rapidly in the wild. As morels age in the wild, residual autolysis causes the release of nutrients which cause undesirable bacteria to grow on the tissue. The undesirable bacteria are called "gram negative" because of a test which shows that they have ancient type cell walls. These cell walls contain lipoproteins (called endotoxin) which chew through the cell walls of other species including plants and animals. When people eat old morels they often get sick due to the gram negative bacteria growing on them. This doesn't happen with other mushrooms. They appear circumstantially to excrete carbohydrates which cause "gram positive" bacteria to grow on their surface and shove out gram negatives. Gram positives are safe to eat, if they don't have some other undesirable features such as disease causing characteristics. Ascospores (spores inside of cells) are very problematic for yeasts and the morel. The morel needs a lot of surface area for ascospores resulting in heavy ridges with deep pits between the ridges. To get the spores out, the tissue must dry and shrink, which creates a force to propel the spores out. These requirements create contradictions which are not totally resolvable. The tissue must stay hydrated for about three days while spores are forming. Then the tissue must dry and shrink before deterioration occurs. To improve the chances of all that occurring, the morel forms some types with thick tissue, to delay drying if needed, and some types with thin tissue, to speed up drying when needed. Thick tissued morels have a narrow base, which slows drying, while thin tissued morels have a wide base, which speeds drying. These differences are called phenotypic rather than genotypic. Phenotypic means the appearance is different while the DNA is the same. Phenotypes growing near each other can be known to be the same genotypes (same DNA) for two reasons. One, gene exchange will homogenize the gene pool making them all approximately the same. And two, two different genotypes cannot occupy the same niche. One will prevail against the other. These morels are all from the same patch, which means they are all genotypically the same, while they are phenotypically different in extreme ways.
Phenotypic variations are extreme with morels due to the difficulty of getting spores out. It's a method of adapting to environmental variations when genetics and evolution are not adequate for the purpose. All species have some phenotypic variation as a method of adapting to changes which are too fast for evolution. Usually, it is seasonal variations which are rapid which require the most phenotypic variation. An example of phenotypic variation with humans is muscle types. There are two types of muscle cells: fast muscles and slow ones. Each person has a different combination of the two types. Persons who have fast muscle cells are good at tennis; and persons with slow muscle cells are good at endurance such as long distance running. These differences are randomly distributed through a population rather than following lines of Mendelian inheritance. The test of phenotypic variation is its random distribution through a population. Morels have so much phenotypic variation that there has probably never been two morels the same. There are probably variable phenotypes for every gene. Variations in the same gene are called allotypes. In studying many of the common enzymes in morels, from three to five allotypes are usually found for each protein or corresponding The morel does not vary phenotypes in a controlled way. It randomly re-scrambles the combination each time a spore is formed. As a result, most of the combinations are nonfunctional. This results in some of the morels being very weird looking. I have found that only about ten percent of the morels in my area are capable of producing functional spores. This would vary from area to area, as phenotypic variation does. When environmental conditions are extreme, as on the hot plains, there is a lot of phenotypic variation. Where weather is more consistently humid and cool, there is less noticeable phenotypic variation. Consider the puffball by contrast. It's the oldest mushroom having something similar to its present form 200-300 million years ago. It is very highly refined. It has four phenotypes that I have noticed. They are each quite different. In other words, it takes much evolutionary time to reduce the phenotypes to essential characteristics. Phenotypic variation shows up in the anomaly of morel mycelium. The anomaly is a combination of tissue cells and mycelium which forms on the surface of agar medium. It is made up of scrambled characteristics of the morel. The anomaly shows variations in colors and shapes, just as the morel mushrooms do. Each anomaly shown here is an outgrowth from a single spore. Each spore outgrowth is a different phenotype. Phenotypic variation is extreme with wildflowers which grow under harsh conditions. The color of the flower petals and their shapes will be highly varied. These pairs of wildflowers are genotypically the same but phenotypically different. They grow near each other, which means they exchange genes and become genetically the same.
Morel mycelium reverts to masses of cells called sclerotia under the ground as a method of surviving harsh conditions which include summer heat and winter cold. The mycelium usually takes more than one year to get adequately developed after a spore germinates. Typically, the mycelium will cover an area of 6-8 feet in diameter. This can be known because the same phenotypes will be that far apart. As the mycelium is expanding over an area, it constantly reverts to sclerotia as conditions get warm and dry and then grows back into mycelium Seldom do mushrooms form sclerotia, because their mycelium is hardier and more able to tolerate variations in conditions. The puffball is again extreme in its ability to tolerate harsh conditions. Its mycelium prefers the hardest, driest ground, such as car trails, because there will be less vegetation there to block spores from escaping into the air. Puffball mycelium will develop over many decades often spreading over hundreds of feet of space. More typically, mushroom mycelium does not survive winter freezing and must be restarted from spores each year. University scientists assume the purpose of sclerotia is to start the formation of a mushroom. This assumption stems from an elaborate growing procedure which was developed by first producing large masses of sclerotia and then placing them in a tray for morel production. In this situation, the sclerotia is serving as a food source with well established mycelium, but scientists claim it is a life-stage cycle preceding mushroom formation. The ridiculousness of that assumption is that sclerotial masses do not get large under natural conditions but are spread thinly over a large area. A mushroom cannot form from these tiny masses of sclerotia, or there would be dozens of them per square foot. The A Garden of Eden In 1994 a Stone Henge-like ritual site was found in western Turkey (3). It's called Gobekli Tepe. It was dated to 12,000 years ago. This is when the last ice age was ending. The environment was a paradise resembling the Biblical "Garden of Eden." Now the environment is rocky and barren. The assumption is that humans destroyed the environment by abusing it. I would theorize that 12,000 years ago, that location was at the leading edge of a glacier. Cold and humid air coming off the glacier created a rapid transition between something like a rain forest and a desert. In between the extremes would have been all imaginable ecosystems. To transition between diverse ecosystems in such a short distance gave humans the ability to exploit the environment to a maximum. The soil looks like glacial till pointing to such a history. The evidence of the morel mushroom evolving in such an environment and the Garden of Eden-like conditions at Gobekli Tepe point to the transitional front of the glacial mass of an ice age creating ideal conditions for evolution and human exploitation. Scientists often encounter phenotypic variation, but they are confused by it. They often assume it is caused by environmental conditions. The cumulative effect of gene exchange is adaptation by improved function. The cumulative effect of phenotypic variation is adaptation by multiple options. The test of phenotypic variation is random distribution of genetic variation through a population. Phenotypic variation can be known to be genetic rather than environmental by the repetition of patterns over and over in a population. It is sustained in a population. It involves factors which must be under genetic control such as morphology and pigmentation, which are products of complex biochemistry under genetic control. The harshest conditions produce the most phenotypic variation. Prairie wildflowers are very extreme in their phenotypic variation due to harsh conditions. Multiple alleles are used to create phenotypic variation. Different alleles are turned on and off during gene exchange. Scientists have known for a long time that multiple alleles exist, but they didn't know why. Creating varied phenotypes is why. The morel mushroom has extreme phenotypic variation, and extremes have been found in alleles. The TCA enzymes were separated and found to have 3-5 allotypes for each enzyme. Phenotypic variation as an adaptation mechanism is an important biological phenomenon which was not previously known to exist. When aware of it, it is easy to see in plants. Presumably, it exists in animals also. It would be common in yeasts, which is where the morel acquired it. The phenomenon is observed as variations in a population which would be genetically homogeneous. Populations become homogeneous over time due to gene exchange. Variations must be introduced from outside sources. The source of differences is diverse environmental conditions. In other words, inbreeding causes offspring to become genetically similar. Inbreeding means lack of outside diversity. In this way, Mendelian inheritance homogenizes the gene pool in the absence of outside sources of diversity. When variations are introduced into a population, they spread according to the Mendelian pattern, which means from parents to offspring with half of the genes from each parent. Gradually, the variations blend uniformly into the population. By contrast, phenotypic variation is observed as randomized distribution of variations, and it does not disappear over time by blending into the population. The test of phenotypic variation is randomization of variations in place of the usual Mendelian variations following lines of inheritance. A random distribution of variations is observed. Biological variations are created by differences in environments. An example would be one type of alligator adapting to a swamp which has a lot of grass, and it eats small prey. A different type of alligator adapts to a large open bay, and it eats large prey. So the two types evolve slight differences in size and so forth. Those differences are valuable for survival under diverse conditions, so they are remixed through gene exchange, when the different types come together. Nature invests heavily in gene exchange (as sexuality) so that differences can be remixed into new combinations for improved adaptability. The flowers on plants have that purpose. Flowers attract insects which carry pollen from plant to plant, so differences can be mixed into new combinations. Animals also invest heavily in sexuality producing dramatic colors and courtship rituals to promote gene exchange, so differences can be promoted for better survival under a diversity of conditions. But gene exchange does not create differences, it can only remix what already exists. The mixing process homogenizes the gene pool. Over time, all offspring become similar, unless new sources of variation are added, and they must come from a diversity of environments. A qualifier must be added here. The remixing of genes during gene exchange does produce individual combinations in new ways, but pre-existing genes must be used. The evolution of new genes is a slow process that occurs in response to variations in environmental conditions. How the mixing occurs is evident in human ethnic groups. A few centuries ago, humans were quite isolated and did not move around much. So ethnic groups acquired identifiable characteristics. When they started to travel to different areas and mix with other persons, the differences started to disappear. Notice that an ethnic group acquires its characteristics because gene exchange homogenizes the group, when external sources of variation are limited. In other words, gene exchange does not create diversity, it destroys diversity. Diversity is generated by varied environmental conditions. A lot of scientists assume otherwise. They assume diversity is a product of gene exchange. An alternative to the process of using gene exchange to remix characteristics is phenotypic variation. It is a mechanism used by yeasts because of their limited gene exchange. Yeasts are fungi which adapted to sugary solutions during the era of modern plants beginning about 65 million years ago. In sugary solutions, yeasts lost the ability to disseminate easily. External spores were converted to internal spores, so single cells could form spores. Then the wind could no longer carry spores from one group of cells to another. The limited gene exchange left yeast with reduced ability to adapt to variations in environmental conditions. So to survive under a variety of conditions which they might encounter, they used phenotypic variations. So what is phenotypic variation? It is a difference in appearance or function for types which are genetically the same. For example, the morel forms thick-tissued and thin-tissued mushrooms to cope with variations in weather. Another example of phenotypic variation is embryonic development. Different types of cells are created, even while they are all genetically the same. A muscle cell is genetically the same as a fat cell. The differences are phenotypic, not genotypic. The way it occurs is by repressing some genes and using others. Each cell type uses a different combination of genes, even though all of the cells have the same reservoir of genes. The morel mushroom evolved from a yeast and carried phenotypic variation from its ancestor. It had to continue to use phenotypic variation, because its ascospores limit gene exchange. The morel spores are formed inside of cells (asci) on the surface of the mushroom. A force propels the spores out of the asci. The force is created by shrinkage of tissue upon drying. The spores are very heavy, which allows them to function better as projectiles. They often contain more than twenty nuclei, which increases their mass. Some spores stay on the surface of morels to be picked up by wind. But wind is not used effectively by morels. The surface of morels lacks the aerodynamic advantages of gills, which allow wind to sweep out a large number of spores; and the morel environment is surrounded by trees and brush which reduce wind and block spore movement. Another problem with dissemination of spores by morels is that there are large amounts of space between localized populations of morels, because the soil must be sandy or similar, which is usually limited to river basins. There is a lot of diversity of morel types, and those differences are readily observed over distances of a few hundred miles. But spores are not easily carried by wind over those distances. Regional differences are sustained for this reason. Otherwise, there would be more homogenization of types, as observed with other mushrooms. So the morel had to continue to use and develop phenotypic variation as an adaptation mechanism. It did that by producing a large number of variants which have the same genotype. Some of those variants are better adapted to a particular set of environmental conditions than others. Here's a subtle point which is the key to understanding this subject. If the morel can produce so many variants phenotypically, why not do it genotypically? After all, there has to be a gene for each phenotype. There are two answers to that question. One is time. Speed is the precious commodity of gene exchange. If genes can be rapidly rearranged into new combinations, then changing environmental conditions can be met with new functions. But if diverse options cannot be rapidly brought together and remixed, then a large number of options must be sustained continuously, which is phenotypic variation. The other answer is that genotypic adaptation involves loss of genes. All of the disadvantageous genes are discarded. But gene exchange allows a complete variety of genes to be reacquired from their original sources of diversity. If however, gene exchange is too limited, genes which are lost cannot be reacquired easily, and then the whole diversity of options must be carried through each generation, as occurs with phenotypic variation. The cumulative effect of gene exchange is adaptation by improved function. The cumulative effect of phenotypic variation is adaptation by multiple options. Improved function is more adequate and complex than multiple options. So the phenotypic variation of Morchella is a disadvantageous necessity resulting from the use of ascospores and a carry-over from its yeast ancestry. Anomalies are rare and informative in science. The morel anomaly defies usual evolution and physiology in an extreme way thereby providing much information. Most species cannot produce extreme aberrations, because their characteristics are highly refined over millions of years. It's only because the morel was a single-celled organism a few years ago that it can produce numerous aberrations. Species usually have a fixed shape (morphology) which cannot vary much. If animals have a birth defect, it can be traced to one step in embryonic development. The morel anomaly is not off by one step; all of its morphology is off and some of its physiology. The moral anomaly occurs when the mycelium grows on the surface of a liquid or gel with optimum nutrition. A detailed nutritional study was required to optimize nutrition. With the anomaly, the mycelium starts to change its morphology and physiology without forming anything resembling a mushroom. Mushroom mycelium can never form pigment, beyond traces of accidental color, because it grows in the dark where pigment is not visible. It takes enzymes and energy to produce pigments. No cell waists energy and physiology producing something it cannot use. But it is not the mycelium per se producing the pigment in the anomaly; it is cells undergoing change which have a microscopic structure. For mushroom cells to grow flat on a surface and produce pigment is an extreme anomaly. It isn't just one thing wrong with the morphology; it is everything wrong with the morphology. The physiology produces pigment when there is no mushroom for the pigment. There are loops and curls in the anomalies which are rudimentary formations of ridges and pits on the surface of the tissue. The tissue in the anomalies partially differentiates into the morphology of the mushroom. One important thing that the anomaly says is that the morphological structure of the morel has not been stabilized the way it is for other species. Other species cannot revert to some other shape, because there is no pattern other than their usual shape. But the morel has a pattern to follow when growing on a flat surface. This means the morel was growing on a flat surface so recently that it still carries the pattern for flat surface growth in its DNA; and the DNA transcription can revert to that pattern. It is surprising that DNA can carry a pattern which can no longer be used. The flat-growth-pattern is in fact integrated into the normal pattern, which scrambles the characteristics of the anomaly. The pigments in the anomaly are characteristic of the normal morel strain. In other words, if the strain has a red pigment, it shows up in the anomaly, and if it has a black pigment, it shows up in the anomaly. When adding this evidence up, it means the morel evolved from a yeast so recently that it can revert back to a yeast-like growth, where there is no macromorphology when growing on a surface. The round shape of yeasts is promoted by growth in liquids, and the filamentous shape of molds is promoted by growth on surfaces. There are two types of evolution. Minor changes occur in a slow and continuous manner with all species. Major changes are made in large leaps which occur rapidly. Both are caused by changes in environmental conditions. Radiation and chemicals produce point mutations for slow change, while flowers and sexuality create large leaps through redistribution of genes. Morel mushrooms are undergoing rapid change, as a yeast growing on trees adapted to the soil. The end point must be a cup fungus for long term survival, because ascospores require that strategy. A cup-like indent is appearing on some variants of the morel, but only a related genus, Helvella, is apt to complete the process. Ascospores are extremely contra-survival for filamentous fungi, which means they only exist when carried from a yeast ancestor. Yeasts and higher fungi evolved in opposite directions creating major differences in many of their important characteristics. Yeasts evolved toward high speed growth through simplicity, while higher fungi sacrificed time for complexity. The basis for the difference is that yeasts must battle more directly against competitors, while higher fungi battle against the elements growing on surfaces in ways which competitors cannot. Yeasts began their evolution about 50 million years ago, as indicated by fossil evidence and circumstances. Modern plants began producing sugary substances at about that time, which is the primary factor causing yeasts to evolve. Sugar is a dehydrating agent, so it must be in the form of dilute liquid to be utilized by microbes. Since competitors including bacteria and molds can grow in sugary solutions, high speed growth was needed by yeasts. To maximize growth rate, yeasts discarded all possible complexity. Besides simplifying morphology, they minimized the number of enzymes they maintained. Discarded were most extracellular enzymes for breaking down large molecules plus enzymes for using unusual nutrients. Of the remaining enzymes, about ninety percent were repressible being synthesized only when needed. The result for typical yeasts was a mass doubling time of about 90 minutes at room temperature compared to 45 minutes for bacteria. High growth rate was not the only adaptation to increase competitiveness of yeasts in sugary solutions. Also important was the excretion of acid and alcohol. Yeasts normally break down sugars into two-carbon compounds through glycolysis, while repression of TCA enzymes prevents further reduction. Some of the carbon is used for synthesis, but a limited amount is excreted as acid and alcohol to inhibit growth of competitors. The excreted carbon also causes sugar to be depleted more rapidly making it less available to competitors. When the sugar is depleted, TCA enzymes are synthesized, and the excreted carbon is remetabolized. Acid and alcohol excretions by yeasts are under intrinsic control, and the quantities depend upon adaptations. While alcohol must be excreted for anaerobic glycolysis, the extent to which such fermentation is used varies with adaptations. This procedure is effective, because yeasts can develop a high tolerance for acid and alcohol. They usually tolerate pH 3.0 well. Bacteria in general do not tolerate acid as well as yeasts, because their environment is not usually acidic, and because their prokaryotic characteristics do not meet the demands as well as does the complexity of eukaryotic cells. Molds have a low tolerance for acid in organic form, at least when it is absorbed. Circumstantially, the reason appears to be that protons must be pumped out of the cell, while molds must avoid excreting substances which concentrate on exposed mycelium and kill it. The evolution of filamentous fungi traces back in fossil records to the beginning of terrestrial life. New forms of fungi continued to originate from different starting points for several hundred million years. Since yeasts appeared much later, they could only have evolved from higher fungi. The diversity of yeasts indicates that they evolved from several different lines of higher fungi. If this assumption is true, there should be more phylogenetic difference between some yeasts than between some yeasts and some higher fungi. There is a window of opportunity for all evolution; and it closes, because basics cannot change much after they are depended upon. Early in evolution, while basics were still alterable, higher fungi acquired specialized characteristics. Yeasts gave up some of those characteristics and cannot regain them, because that type of evolution no longer occurs. The demanding evolution which higher fungi underwent created the following characteristics: 1. external spores Yeasts gave up those characteristics, and it appears that they cannot reacquire them. Some yeast-like fungi have external spores, but they would have carried them from ancestors. Yeasts maintain some extracellular enzymes for breaking down polysaccharides. (It is most likely that yeasts release such extracellular enzymes through autolysis, as bacteria do, rather than synthesizing them on the cell surface, as filamentous fungi do.) Yeasts not only excrete in a controlled manner but also excrete a variety of metabolites in a manner which appears to be inadvertent. The uncontrolled excretion indicates a lower efficiency of metabolism than higher fungi have. Influencing metabolic efficiency is the spatial arrangement of enzymes which are attached to membranes, and this characteristic would be resistant to change. There is no evidence of filamentous yeasts tolerating dehydration. For example, when insects make holes in trees causing sap to be exuded, the filamentous yeasts grow in the bore holes, where there is protection from dehydration, while unitary yeasts grow in the exudate on the bark. Like yeasts, Morchella mycelium lacks resistance to dehydration. When Morchella is cultured, exposed mycelium dies off and turns brown, unless humidity is saturated. When Morchella mycelium turns brown from dehydration, it excretes alkali, which would primarily be ammonia resulting from residual autolysis. (The alkali is observable on the mycelium with color indicators.) Yeasts and bacteria undergo autolysis upon die off, which allows nutrients to be recycled. Molds do not. Morchella evolved from a yeast so recently that it continues with a degree of autolysis which is not advantageous. There is evidence of a disadvantage for Morchella autolysis. Deliquescence is a problem for Morchella. Bacterial attack appears to be involved, as the breakdown is spotty. Also indicating bacterial attack are incidents of long time morel eaters getting sick on morels. The cause in some cases would be endotoxin from gram negative bacteria growing on the surface. Gram negatives would attack the tissue, while gram positives would not; and gram positives would be edible. Considering the viscous surface of other mushrooms, they probably promote the growth of gram positives by excreting a small amount of carbohydrate. Morchella apparently promotes gram negative bacteria by excreting nitrogen through residual autolysis. Residual autolysis may stem from some of the autolytic genes being involved with differentiation preventing them from being discarded until new genes are produced for that purpose. Yeasts do not adapt to soil growth. Yeasts are found in the soil, but their prevalence is sporadic indicating that they are there by chance. The main problem for yeasts adapting to the soil is dissemination. Normally, they are disseminated by insects which feed on them and pick them up on their feet. The extent to which air or water disseminates yeast apart from insects is unknown. While the ancestor of Morchella began adapting to soil growth, it needed to continue with spore dissemination above the ground utilizing insects and eventually wind, before an ascocarp was developed for that purpose. During this phase of its evolution, surface exposure would have created vulnerability to dehydration. Therefore, the weather must have been consistently rainy or very humid. Once the yeast ancestor of the morel acquired the ability to evolve in the soil, it was drawn in because of the exploitability of bacteria as nutrients. Typical yeasts inherently have the ability to feed on bacteria by killing them with acid, though the quantity of bacteria in their environment is not usually great. The soil contains a large number of bacteria early in the spring due to recycling of nutrients. Freezing provides nutrients for microbes by breaking apart cells and releasing their contents when the ground thaws. The most specialized bacterium for exploiting cell debris in spring soil is Pseudomonas fluorescens (P.f.). Its name stems from a water-soluble fluorescent pigment which it produces. The purpose of the pigment is to attract insects for dissemination, much like the color of flowers. It releases proteolytic enzymes into the environment through autolysis, and the enzymes break down proteins from the cell debris. In a study of P.f., this author found that it undergoes complete die-off sharply below pH 5.0. This pH appears to be an adaptation designed to release enzymes and recycle nutrients. It means P.f. is highly exploitable by a yeast which can enter the soil and excrete acid. The exploitability of P.f., and perhaps other bacteria, appears to be the primary factor which allowed Morchella to adapt to soil growth in spite of its other characteristics being poorly suited for the purpose. Normally, mushroom mycelium would not be expected to excrete acid, because the acid would concentrate on the mycelium and kill it. Morchella, however, appears to have become dependent upon the excretion of acid for attacking bacteria and feeding on them. To use acid most effectively, it appears that Morchella repeatedly reabsorbs it, channels it through the mycelium and re-excretes it. This mechanism causes the acid to accumulate in absorbent areas while not accumulating on the mycelium. Laboratory evidence of this mechanism is found in nitrogen utilization. In most, if not all, cases, fungi absorb ionic nutrients without their homologous ion, which results in a shift in exogenous pH. When ammonium ions are used as a nitrogen source by Morchella, the medium becomes extremely acidic stopping growth at about pH 3.0. To start growth on ammonium ions, the initial pH must be 6.0 or higher; and even then, mycelial growth is thin. If, however, an alkali creating nutrient is used with ammonium ions, the initial pH can be much lower, and mycelial growth is much thicker. Examples of alkali creating nutrients are K-acetate and Na-glutamate. K-acetate produces a strong rescuing effect for growth on ammonia, because it neutralizes the acid that forms on the surface of the mycelium. Na-glutamate is used more slowly and is thereby less effective. The difference between initial pH and developed pH is that newly inoculated mycelium is not in an ideal condition and must undergo adaptation to the new medium. The adaptations create a heavy drain on the ATP supply. ATP appears to be needed for pumping protons out of the cell but not for absorbing them, as known to be the case with yeast. During initial growth, it appears that protons are freely absorbed but cannot be pumped out because of a shortage of ATP. Therefore, initial growth requires a high pH, or the acidity must be neutralized on the surface of the mycelium through an alkali creating nutrient. Requiring an initial pH of 6.0 for utilization of ammonia indicates a developed tendency for Morchella mycelium to absorb protons as a method of protecting the mycelium from acid. Evidence of Morchella mycelium excreting acid is found in agar media, when using a nitrogen source which does not apparently alter pH, such as casein hydrolysate. The amount of acid produced is proportional to the dextrose concentration indicating that the acid originates with the dextrose, and therefore it would be acetic acid. The amount of acid is not great, as it reduces the pH from 7.3 to 6.0 in typical agar media with moderate buffering capacity. A small amount of acid can be used quite effectively in attacking bacteria by causing it to concentrate where it gets absorbed. So the nutritional evidence combined with the ecology strongly indicate that Morchella attacks bacteria with acid and feeds on them. Morel mushrooms have a bulbous shape which maximizes surface area for the emission of ascospores. Ridges on the surface also increase surface area. Wind currents are not used well for dissemination of ascospores, because a force propels the spores from the ascus independent of wind currents. A few spores remain on the surface to be picked up by wind currents; however the morel cap does not protect the spores from being washed to the ground by rain.
Another limitation to dissemination of Morchella spores is heavy vegetation in the environment resulting in wind currents being diminished and spores being caught by the vegetation. Morels emerge in the spring when there is usually little wind beyond sporadic storms.
Considering these factors, spore dissemination is highly unreliable for Morchella, so much so that spores are not depended upon for seasonal survival. Instead, sclerotia serves that purpose. Sclerotia functions as a resistance state for survival of mycelium under harsh conditions. Apparently, modern (gilled) mushrooms must rely upon spores for survival through winter conditions, because the mycelium will not tolerate freezing. Their spores do not usually germinate readily because of their need to survive through the winter. Morchella spores do, however, germinate readily, because they cannot be used reliably for seasonal survival. They are used for dissemination only. Presumably, the ancient mycorrhizals, such as boletes, have hardy mycelium which will survive freezing. Truffles might not be so ancient, and the inability of the mycelium to survive freezing may be why they form a mushroom underground. The morphology of thick-tissued morels is highly informative. Morels growing in the upper plains of U.S.A. are exposed to hotter and dryer conditions than usual. They have therefore been recently adapting by producing a thicker-tissued variant. Thick-tissued mass has been evolving so rapidly and recently on those morels that it has a tendency to be disordered. This effect shows up as globs of tissue hanging off the cap at the base or oversized ridges creating bumps or globs of tissue. There are thick-tissued morels from other areas which have a more refined morphology. Another feature of the thick-tissued morel is an indent in the cap, which slows the rate of dehydration even further. At the indent, ridges are closer and tissue more concentrated creating a slow-drying area on the cap. This indent would be the early evolution of a "cup fungus." It demonstrates the function of the cup which ascosporogenous fleshy fungi usually have. The cup collects water and creates an aggregation of tissue at the bottom while leaving thin and exposed tissue near the rim. The cup thereby maximizes the extremes between slow drying and fast drying tissue. For these reasons, the cup shape is the form toward which fleshy ascomycetes must usually evolve, and there is a convergence of evolution at that point. Of course, the "cup" shape has numerous variations. Morchella would have to evolve into a cup fungus to survive through more than one ice age, which means it is only one ice age old. The recent ice ages have been cycling at 100 thousand year intervals for the past million years. The yeast which Morchella evolved from and the ecology would not change much in such a short amount of time; so the same starting point for Morchella evolution appears to redevelop at the beginning of each ice age. There are cup fungi said to be closely related to Morchella based on spore morphology (Nancy Smith Weber, A Morel Hunter’s Companion, 1988). They include Disciotis venosa, Discina leucoxantha and other Discina. Appearing in the spring in an environment similar to that of Morchella but being slightly more advanced than Morchella, they appear to have evolved from the Morchella line during an earlier ice age. Cup fungi vary somewhat in their shape. If they need to dry easily, they have thin tissue; and if they need to delay drying, they have folds in them. The Morchella cap is quite impractical for survival under adverse conditions. The cap demonstrates extravagance in producing a large but simple structure which does not tolerate much variation in weather. Such an impractical cap is made possible by the use of sclerotia for seasonal survival, because then ascospores do not have be reliably produced every year. There is much information about the characteristics of Morchella revealed in other mushrooms which evolved with it, which appears to include the families Helvellaceae and Morchellaceae. A very informative example is Helvella crispa. This mushroom is smaller than the morel. It emerges in the same environment as the morel but about a month later, when weather is hotter and dryer. Its cap is shaped like a potato chip hanging over a stalk. Its ascospores do not germinate readily indicating that they are used for seasonal survival, and sclerotia can be assumed to not be produced. Helvella crispa’s small size and absence of sclerotia would stem from a diminished availability of bacteria to feed upon late in the spring. Since it cannot rely upon sclerotia, it must effectively emit spores every year. To achieve this, its cap is much like that of a cup fungus, except that it does not collect water. There is an aggregation of tissue near the stalk, while tissue is thin around the outer edge. Somewhere in-between, drying occurs at the right rate each year. This comparison indicates that Morchella splurges on the shape of its cap, because it can produce sclerotia from the abundance of nutrients which are available early in the spring. But the dependence upon sclerotia limits it habitat to that which can produce a large quantity of bacterial nutrients. The cup fungi have a broader habitat, because they are not dependent upon such rich nutrients for sclerotia production. The cup fungi which are related to Morchella thereby appear to be more similar to Helvella than Morchella, at least in terms of the described characteristics, and therefore they may have evolved from that genus. These types should re-evolve during each ice age along with Morchella. The cup fungi related to Morchella indicate a re-evolution of types with each ice age. There are numerous obstacles for a yeast to evolve at the base of trees at this time. Normally, tree exudate would be needed for nutrients, but it is sporadic. Dehydration would be a problem when there is not a lot of exudate. Perhaps cold, humid air sweeping off the ice sheet creates an improvement upon tree sap at the base of trees. Normally, only elm trees produce a large amount of exudate, and it is sporadic. However, conditions under the loose bark of some trees create suitable conditions for yeasts. The yeast genus Nadsonia has a species, elongata, which is adapted to growing under the loose bark of birch trees. This type of growth may be improved in front of the ice sheet. Indications of Morchella evolving from a yeast are these: 1. The reversion anomaly lacks normal structure. ------------- 1. O'Donnell K, Rooney AP, Mills GL, Kuo M, Weber NS, Rehner SA (Mar 2011). "Phylogeny and historical biogeography of true morels (Morchella) reveals an early Cretaceous origin and high continental endemism and provincialism in the Holarctic". Fungal Genetics and Biology 48 (3): 252-265. doi:10.1016/j.fgb.2010.09.006 . PMID 20888422 . 2. Thomas N. Taylor and Edith L. Taylor. The Biology and Evolution of Fossil Plants. 1993. Prentice Hall, Englewood Cliffs, New Jersey. 3. Gobekli Tepe. Tom Knox. Do these mysterious stones mark the site of the Garden of Eden? March 5, 2009, Daily Mail (MailOnline). |


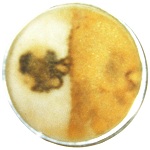 The ability to revert to such a structure indicates a recent history of multicellular surface growth in nature.
The ability to revert to such a structure indicates a recent history of multicellular surface growth in nature.
 Different parts of the DNA are used thereby showing different characteristics. Genotypic differences are those resulting from differences in the DNA, as occurs with inheritance. Tissues are examples of phenotypic differences, where the DNA is the same in all of the tissues, while different parts of it are used for each different tissue. The areas on the DNA chromosomes which are not being used are covered with proteins. Each type of tissue has different areas on the DNA covered with protein. This is why stem cells are needed to create tissue. Stem cells have all of their DNA freely exposed.
Different parts of the DNA are used thereby showing different characteristics. Genotypic differences are those resulting from differences in the DNA, as occurs with inheritance. Tissues are examples of phenotypic differences, where the DNA is the same in all of the tissues, while different parts of it are used for each different tissue. The areas on the DNA chromosomes which are not being used are covered with proteins. Each type of tissue has different areas on the DNA covered with protein. This is why stem cells are needed to create tissue. Stem cells have all of their DNA freely exposed.
 gene.
gene.
 By contrast, the domesticated flowers evolved under ideal conditions and do not show much phenotypic variation.
By contrast, the domesticated flowers evolved under ideal conditions and do not show much phenotypic variation.
 after rains.
after rains.
 morel must evolve into a cup fungus for long term survival. An indent in some of them shows the beginning of evolution in that direction. The cup shape combines thick tissue and thin tissue. Thick tissue is near the ground and slow at drying. Tissue gets thinnest at the rim, where rapid drying can occur. Supposedly, somewhere in between drying occurs at the right rate each year for getting spores emitted. But it is a losing battle. The cup fungi last two ice age cycles and die out. They are in two genera which include Disciotis venosa and Discina leucoxantha. They are said to have identifying features on the surface of the spores which are the same as on the morel (Nancy Smith Weber, A Morel Hunter’s Companion, 1988).
morel must evolve into a cup fungus for long term survival. An indent in some of them shows the beginning of evolution in that direction. The cup shape combines thick tissue and thin tissue. Thick tissue is near the ground and slow at drying. Tissue gets thinnest at the rim, where rapid drying can occur. Supposedly, somewhere in between drying occurs at the right rate each year for getting spores emitted. But it is a losing battle. The cup fungi last two ice age cycles and die out. They are in two genera which include Disciotis venosa and Discina leucoxantha. They are said to have identifying features on the surface of the spores which are the same as on the morel (Nancy Smith Weber, A Morel Hunter’s Companion, 1988).  It means a new genus forms during each ice age, and only two genera means two ice ages. This isn't the result of 129 million years of evolution claimed by university scientists. It's the re-evolution of the morel during each ice age cycle.
It means a new genus forms during each ice age, and only two genera means two ice ages. This isn't the result of 129 million years of evolution claimed by university scientists. It's the re-evolution of the morel during each ice age cycle. It forms visible pigment and a variety of cell structures at the microscopic level, while it forms a flat sheet on the surface.
It forms visible pigment and a variety of cell structures at the microscopic level, while it forms a flat sheet on the surface.
 Morel ascospores are very large and heavy, containing numerous nuclei each, which causes them to sink in water in a matter of seconds. They therefore travel as projectiles, as demonstrated by the distribution of spores around morels which are drying on a surface. This means the spores have no ability to be suspended in air without wind.
Morel ascospores are very large and heavy, containing numerous nuclei each, which causes them to sink in water in a matter of seconds. They therefore travel as projectiles, as demonstrated by the distribution of spores around morels which are drying on a surface. This means the spores have no ability to be suspended in air without wind.
 Also limiting spore dissemination is a failure of morels to come up every year in areas where spring weather can be dry. Growth is sustained under the ground from year to year in the form of sclerotia, which is a brittle mass of spore-like cells.
Also limiting spore dissemination is a failure of morels to come up every year in areas where spring weather can be dry. Growth is sustained under the ground from year to year in the form of sclerotia, which is a brittle mass of spore-like cells.
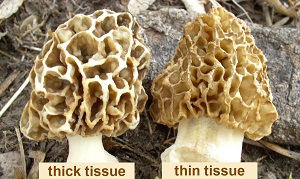
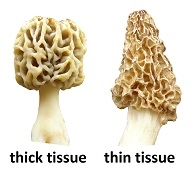 The ascocarp is hollow, which promotes drying. Tissue thickness is controlled primarily by the width of ridges and the amount of space between them. With thick-tissued types, ridges are wide and curved with little space between them. With thin-tissued types, ridges are small leaving much space between them. Another identifying difference is the width of the stem, which correlates with the amount of empty space inside the cap. Thick-tissued morels have a narrow stem, while thin-tissued ones have a wide stem. These factors control how concentrated the tissue mass is and therefore its rate of dehydration.
The ascocarp is hollow, which promotes drying. Tissue thickness is controlled primarily by the width of ridges and the amount of space between them. With thick-tissued types, ridges are wide and curved with little space between them. With thin-tissued types, ridges are small leaving much space between them. Another identifying difference is the width of the stem, which correlates with the amount of empty space inside the cap. Thick-tissued morels have a narrow stem, while thin-tissued ones have a wide stem. These factors control how concentrated the tissue mass is and therefore its rate of dehydration.