|
|
Gary Novak
The Cause of Ice Ages and Present Climate |
pH in the Oceans Explained
Hydrogen ions determine pH. A hydrogen ion is simply a proton, which has a charge of plus one. The numbers for pH are the exponents for the base ten with a minus sign which is not shown. Neutral is pH 7. pH 7 means 10-7. pH 8.1 means 10-8.1. Written out, it is this: 0.0000001 and 0.0000000079. Removing 6 zeros from each, it's 0.1 and 0.0079. Dividing shows 13 times as much acid at pH 7 compared to pH 8.1. This means there are about one thirteenth as many hydrogen ions in the oceans as in neutral water. Oceans would need to be 13 times as acidic to be neutral.
Calcium carbonate is an unlimited buffer in the oceans for two major reasons. One, there is a huge amount of undissolved calcium carbonate in mineral form in ocean sediments including coral reefs. Two, there is a huge amount of dissolved calcium which is only partially associated with carbon dioxide in its various forms of carbonate. Sea water has five times as many calcium molecules as CO2 related molecules, and there are an awful lot of CO2 related molecules. An organization called "Seafriends" does a lot of research on ocean chemistry and states that "...the concentration of Ca (411ppm) is 10.4 mmol/liter and that of all CO2 species (90ppm) 2.05 mmol/liter..." Dividing 10.4 by 2.05 means there is five times as many calcium molecules dissolved in sea water as CO2 related molecules. This means sea water could absorb five times as much CO2 and still be highly buffered by calcium. This also means there is not the slightest bit of acidification occurring in the oceans at this time, was not in the recent past, and will not be in the foreseeable future. Yet frauds claim the oceans are getting acid and already killing sea creatures. It's a total fraud. Scientists do not make such claims on the basis of actual measurements; they base it on someone's guess about the past. There were no measurements in the significant past. The most recent fraud is acidification in the area of the Bering Sea due to ice melting. The water is more acidic where ice melts. The reason is because ice is almost pure water. It has no buffers within it, and it starts at pH 7. Melt water absorbs CO2 from the atmosphere and becomes more acidic. Mixing with other sea water normalizes it somewhat. This sort of acidification always happens when ice melts. Humans have very little influence over the result, because they only add 1% as much CO2 to the air each year as already in the air. This type of acidification is not very harmful, because there is no buffer with it, which means even if sea creatures are exposed to it, they easily overcome it. And pH 7 is never going to harm anything in the oceans. Ice melt is always mixing with other sea water, which brings its pH rapidly back to buffered pH 8.1. Yet, oceanographers are describing a panic situation, with sea creatures dying. The most significant fact about such claims is that they are not scientific. Miniscule effects which normally occur all the time are blown up as disasters, the real causes are not determined, known science is contradicted in the claims, the standards of modern research are a joke to a point of fraud and incompetents who cannot produce higher standards have been shoving out real scientists to a point that there is no reliable science left in most areas. This situation shows up in the communication processes. The frauds cannot make scientifically correct statements or produce reasonable rationality. All they can produce for communication is subjective propaganda which has no place in scientific subjects. pH 7 (which never actually exists on any scale in the oceans) will never harm sea creatures, because all eukaryotic cells have hydrogen ion pumps for moving hydrogen ions around and controlling pH. Seashells are living tissue, like teeth, constructed to withstand adverse conditions, while pH 7 is neutral and not adverse. Seashells have proteins and numerous minerals within them to withstand such conditions. Precipitated calcium carbonate does not exist in biology, though some bacterial excrements will create it, which is irrelevant to biology. Some scientists said corals will give up their algae symbionts at small changes in pH; but later studies showed that other symbionts replace them. How could it be otherwise, with a billion years of biology going through hell and back. To claim such miniscule effects are disasters is a mockery of biological history. Oceans scientists are claiming that shells are being damaged by some contrived acid effect, but it is nothing but fake science, including fake tests which do not represent nature and unknown causes which are not pinned down—all in conflict with 500 years of developed scientific knowledge.
|

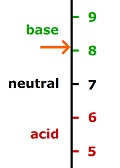 The reason why oceans are always and invariably pH 8.1, unless highly unusual conditions exist, is because calcium carbonate is a strong buffer which holds pH at that level, and there is an unlimited supply of that buffer in the oceans. A buffer is something which absorbs acid and base without allowing much change in pH. It occurs, because some molecule can acquire different forms, thus absorbing acid or base without allowing pH to change much.
The reason why oceans are always and invariably pH 8.1, unless highly unusual conditions exist, is because calcium carbonate is a strong buffer which holds pH at that level, and there is an unlimited supply of that buffer in the oceans. A buffer is something which absorbs acid and base without allowing much change in pH. It occurs, because some molecule can acquire different forms, thus absorbing acid or base without allowing pH to change much.