|
|
Research Photo Pages
|
A mechanism of variation and adaptation which is phenotypic rather than genotypic was found with the morel mushroom. Two types of Morchella variation were studied—spore strain variation manifested through an anomaly on petri plates, and differences in ascocarp tissue thickness as a method of coping with weather. A phenotypic origin for the variations was indicated by functionality and a distribution which was random rather than Mendelian. Visible examples of stable variations in interbreeding populations indicate that this mechanism is widespread in the plant and animal kingdoms. Phenotypic variation as an adaptation mechanism was not previously known to exist in biology. It is manifested as stable variations which are distributed randomnly in an interbreeding population, where a Mendelian distribution and homogenization of variations would otherwise be expected. Gene exchange homogenizes the gene pool for an interbreeding population. The dangers of inbreeding demonstrate this principle. Specifically, half of the genes of parents are discarded during meiosis. Recombining genes while discarding half each time is a process which homogenizes the gene pool. Variations then come from outside sources. They are generated through mutations being acted upon by environmental conditions to select for modified genes which are adapted for survival functions. When they enter a population through breeding, they show a mixture of types only until sufficient gene exchange occurs to homogenize the gene pool. Morel mushrooms (Morchella spp.) have the unusual characteristic of producing variations between spore strains from the same ascocarp. Gene exchange has been assumed to be the source of the variations. However, this study shows evidence that the variations are phenotypic but not genotypic. Morphological manifestations of the variations are shown through differentiation demonstrating that the variations are functional. Evidence of the variations interacting with environmental effects is analyzed indicating that Morchella uses phenotypic variation without genotypic variation as a method of adapting to environmental conditions, and the spore strain variations appear to have this function.
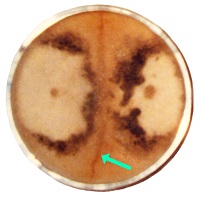
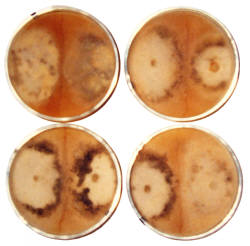
Spore strain variation was first reported as two types of mycelial growth and a reaction zone which develops between mycelia of most spore strains from the same ascocarp (Hervey et al., 1978). The narrowness of the reaction zone (1-2 mm wide) indicates that such cells do not compete significantly with original mycelium. This evidence indicate that the reaction zone functions to wall off diverse cells which exchange nuclei, so they do not dilute phenotypic variations.
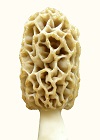
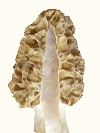
In this study, visible manifestations of spore strain variations were found in high density mycelium (Fig. 7), which is an anomaly consisting of a rudimentary formation of a morel mushroom on agar media. No one else produces such anomalies, because nutrition needs to be optimized and mycologists do not know how to study the nutrition of fungi, as they study in botany rather than microbiology departments. Variations were visible in the size and form of the high density mycelium and pigmentation. These variations in growth and differentiation demonstrate functionality for spore strain variations. A barrier forms between mycelia of different spores strains, which prevents phenotypes from mixing. This phenomenon was first observed by Hervey et al., 1978, but they did not understand the function. They referred to the barrier as a meld. Another example of functional variations which was found in this study appeared in ascocarp morphology and its relationship to dehydration of the tissue. Dehydration and shrinkage of tissue create the force which propels spores from the morel ascus as evidenced when drying morels on a surface. Spores are propelled outward only after much dehydration and shrinkage of tissue has occurred. Drying morel tissue releases spores due to shrinkage which creates a force. In the wild, deliquescence sometimes occurs before dehydration, because morels emerge in the spring while conditions are often wet and cold. Deliquescence before dehydration is a failure to effectively emit ascospores. Morel mushrooms are hollow, which promotes dehydration, but the absence of extra tissue creates vulnerability to an opposite effect. Morels can emerge and dehydrate before spores are formed. To cope with these contradictions, Morchella produces variants with differences in tissue thickness, as shown in Fig. 8. In this example, thin-tissued morels were light brown, and thick-tissued ones were gray. Morphological differences were visible around the base of the cap and in the density and curvature of the ridges. Tissue mass was often disordered on the cap of thick-tissued morels. In-between types were also found. These two types of morels were found in the same environment emerging at the same time. Both extremes were observed large and old indicating that one type does not grow into the other. They would not occupy different niches, because morels are not highly niche specific. The differences in tissue thickness indicate that these variations are a method of coping with varied weather conditions which create a need for thick tissue during dry years and thin tissue during wet years. With weather creating a selection pressure, one type would prevail, and the other would die out, if the differences were genotypic. The differences must therefore have been phenotypic only. This evidence indicates that Morchella uses phenotypic variation without genotypic variation as a method of adapting to varied environmental conditions. Such a method of adapting is not highly effective, because inferior alternatives must be produced along with the more advantageous ones. The rudimentary nature of the mechanism is apparently a consequence of recent evolution from a yeast. The question then is whether the adaptive variations originate with spore strain variations. Close parallels between the unusual variations (both showing variations in populations which would usually appear as homogeneous) indicate that they are the same. All functional variations (as demonstrated for spore strain variations) must be adaptive variations, unless they are random and transitory, because selection pressures would act upon all differences. No other source besides spore strain variation is apparent for the adaptive variations related to tissue thickness. Routine functions such as adaptive variations cannot be controlled through gene exchange because of its random and transient nature. Gene exchange produces variation through a separation of alleles resulting in some alleles getting lost, while genetic homogenization is promoted. In a closed system (lacking external sources of diversity), all allele types but one would eventually be lost through exchanges. Loss of alleles would apparently preclude heterokaryosis as a source of adaptation by variation as exhibited by Morchella. Gene exchange produces adaptation by evolution, not by variation. Evolution produces adaptation by improved function, which involves eliminating all of the variations but one (the essence of natural selection). Therefore, evolution cannot alternate between alternatives, as the variations of Morchella do. The phenotypic variation or spore strain variation demonstrated by Morchella apparently results from alleles being turned on or off in a semistable manner during ascospore formation. There should therefore be local populations of morels in which all members are approximately genotypically identical while maintaining spore strain variations. This mechanism explains some of the visible variations which are found in local populations of morels. Such differences could not previously be explained but were sometimes thought to be due to environmental effects. A source of confusion with morels is that similar morels vary from area to area in their visibly stable characteristics. Small differences and large are observed in the stable variations, and a consequence is taxonomic confusion for the genus. This situation is apparently due to recent evolution, because time should reduce the number of stable variants. A large influence by environmental effects appears to contribute to the diversity by causing genetic variations to develop in response to habitat variations. Morchella appears to be highly influenced by environmental effects due to its yeast-like characteristics which are adapted for growth in sugary solutions rather than production of mushrooms in the soil. The disordered tissue on thick-tissued morels (Fig. 8) indicates a recent adaptation to hot dry weather in its upper plains environment. Recent means during the past 1-5 thousand years. The rapid change and high diversity of Morchella types indicates that Morchella has been evolving for the duration of one ice age only. Ice ages have cycled at one hundred thousand year intervals for the past million years. Hervey, A., Bistis, G. & Leong, I. (1978). Cultural studies of single ascospore isolates of Morchellla esculenta. Mycologia 70, 1269-1274.
|