 |
|
Research Photo Pages
|
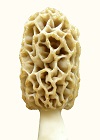
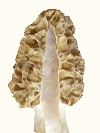
Mycelium of Morchella esculenta and Morchella angusticeps differentiated on agar media creating an anomaly, when suitable nitrogen sources were used. The anomaly was a disc of high density (felt-like) mycelium containing ascocarp pigment and having a rubbery, brittle tissue, similar to that of the ascocarp, below it. The formation of the anomaly demonstrated rudimentary control of morphology and indicated a reversion to a primitive evolutionary form resulting from recent evolution from a yeast. With petri plate cultures and suitable progression of differentiation, ascocarp pigment appeared within the high density mycelium. Morchella esculenta produced gray pigment, but only when a cutout was made in the high density mycelium, as resulted from subculturing (Fig. 1). The need for a cutout indicated that increased aeration was stimulatory to pigment production and the progression of differentiation. With that mycelial type, an orange pigment sometimes formed below the gray pigment (Fig. 2). On day 11, the white disc had a diameter of 82 mm. On day 16, a high density brown disc had a diameter of 65 mm and a boundary around it, while the outer mycelium was thin. The shrinkage of disc diameter, development of a boundary and thinness of extended mycelium are evidence of cell material migrating from the outer mycelium to the high density mycelium in a endotrophic manner. The cell structure of the high density mycelium was filamentous on the upper surface, with cell density increasing toward the agar. On the agar surface below the high density mycelium, a tissue formed having a rubbery, brittle texture similar to that of the ascocarp. The cells extending farthest above the high density mycelium were long and straight with few septa. The septa became more closely spaced at lower depths breaking ultimately into separate polymorphic cells which aggregated into tissue. The agar provided a support base for a layered structure indicating an adaptation to differentiation on a surface. High aeration at the surface along with rich nutrient availability appeared to create the conditions which promoted differentiation. The onset of differentiation could not be determined. The most objective criterion for differentiation was boundary formation. The differentiated mycelium maintained viability for 4-6 wk from inoculation reforming exotrophic mycelium when subcultured. Mycelium exposed on an agar surface deteriorated due to dehydration and toxic effects requiring frequent reisolation. The formation of a tissue, as found on agar, would not be advantageous during normal growth, which demonstrates that the differentiated mycelium was an anomaly. Considering the structured nature of the growth, including zoned ascocarp pigment and a tissue, the high density mycelium appeared to be a rudimentary formation of a mushroom as it existed earlier in evolution. The primitive morphology including a lack of strict controls indicates evolution from a yeast. The ability to revert to a primitive form indicates that the evolution was very recent. Such a formation could have been found at the base of trees during the cold conditions of an ice age, which would reduce dehydration. The base of trees could provide soluble nutrients as exudate and an interface between above and below ground growth for the transitional state of evolution. The likely genus from which Morchella evolved is Schizosaccharomyces based on morphology. The genus Schizosaccharomyces, contains filamentous yeasts and sometimes shows 8 spores per ascus, as does Morchella. In this test, the boundary, being an objective criterion for differentiation, formed only where the agar depth was moderately thin, which meant around the inoculum when placed on the upper half of the slant but not when placed lower down. The agar depth determined the amount of nitrogen available and its rate of depletion indicating that depletion of nitrogen was stimulatory to differentiation. A similar effect was observed with slanted petri plates. The boundary was sharp where agar was thin, but it became invisible where agar was thick. The partial influence of nitrogen depletion indicates that the direct inducer of differentiation was an energy peak which was promoted by inhibited synthesis due to nitrogen deficiency. This mechanism was found to control the sporulation of yeasts, both exotrophic (Croes, 1967) and endotrophic (Novak, 1981), where the physiology has been studied in some detail. Evidence indicates, as described below, that endotrophic differentiation controlled by the energy level is the method by which soil mushrooms in general form. With yeasts, the test of endotrophism is sporulation in distilled water (Novak, 1981). In this study, the test was differentiation on thin agar, where nutrients were depleted. The general mechanism of endotrophism for mushroom formation would be the accumulation of cell mass within the mycelium before it is transferred to the sporocarp as nutrients, preformed molecules and cell components. Croes, A. F. (1967). Induction of meiosis in yeast. II. Metabolic factors leading to meiosis. Planta (Berl.) 76, 227-237.
|



