|
| Gary Novak
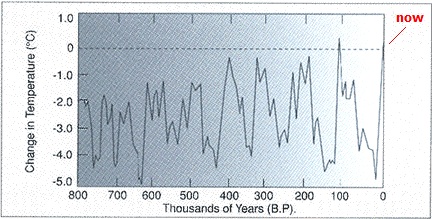
The Cause of Ice Ages and Present Climate |
Ice is not Melted by Air, as the Numbers Show Air does not have enough heat capacity to heat water or melt ice over a large area.
An increase in air temperature does not heat oceans or melt ice which floats. Anyone who studies science should know this; yet carbon dioxide in the atmosphere is said to be heating the oceans and melting Arctic ice. First, air has such a low heat capacity that it doesn't hold enough heat to influence water or the ice within it significantly. Secondly, heat moves upward, not downward, which prevents it from moving from air to water or ice on water. Why would the tropics be warmer, when air does not absorb much sunshine? The atmosphere is nearly the same in the tropics. It's because the water and land absorb the sunlight and transfer the heat to the atmosphere, not the other way around. The numbers show the absurdities. Here are calculations for heating Arctic ice and the amount of globally warmed air required to melt it. These simple numbers are found in numerous places on the internet. The amount of Arctic ice is said to be 12x106 km2 (square kilometers). In the winter of 2010 it is said to be two meters thick, though in some areas it would be thicker. So we will calculate the amount of heat required to melt that much ice when two meters thick. Converting to meters results in one million square meters per square kilometer; and two meters thick results in 24x1012 m3. The density of ice is slightly less than water, or 917 kg/m3. Multiplying these two numbers yields 22x1015 kg of ice. The heat required to melt the ice is called heat of fusion. It is 334 kj/kg. That's kilojoules per kilogram. Multiplying this times the number of kilograms yields 7.4x1018 kj. So how much air would have to be heated 0.6°C (the supposed global warming caused by humans, lately sliding up to 0.7°C, even though global cooling has been occurring) to provide this amount of heat? The heat capacity of air is about one fourth that of water on a gram basis, which is 1 kj/kg-K for air. That's one kilojoule per kilogram per degree Kelvin or centigrade. For 0.6°C, it's 0.6 kj/kg. To get 7.4x1018 kj would require that many kilograms for one degree and 0.6 times as much for 0.6°C, which is 4.4x1018 kg of air. The density of the atmosphere is 1.2 kg/m3 for the first kilometer of height. So we divide the 4.4x1018 kg by 1.2 kg/m3 to get the number of cubic meters, which is 3.7x1018 m3 of air. There are a billion cubic meters per cubic kilometer, so this number is reduced to 3.7x109 km3. This is the amount of square surface area of atmosphere, one kilometer thick, which will hold the necessary amount of heat to melt all Arctic ice when two meters thick. It's 3.7 billion square kilometers of atmosphere. That's 61,000 kilometers on each side of a square. The total area of the earth's surface is only 510x106 km2, which is 23,000 kilometers on a side. The required amount of air is 7.25 times the total amount on the earth. Notice that the surface area of the ice was 12x106 km2, while the surface area of the air for melting it was 3.7x109 km2. The surface area is 308 times as much for the air as for the ice. This means that even if the ice is not all melted, whatever portion is melted requires 308 times as much air per surface area as ice. If one square kilometer of ice is melted, 308 square kilometers of air must be moved over it with all 0.6°C of AGW heat removed. There are an infinite number of complexities stemming from the fact that no one can describe exactly what is happening or produce a consistent theory for what is supposed to happen. For example, where is the heat supposed to be located? The 0.6°C is supposed to be near the earth's surface. How much heating is there supposed to be in the higher atmosphere? None can be detected, yet it is supposed to be back-radiating to heat the surface. Not all of the Arctic ice has been melted, but alarmists claim that it will. If all of the atmospheric heat caused by humans were circulated to the Arctic and melted ice, there would have to be 7.25 times as much heat, which is 0.6 x 7.25, which is 4.35°C. But much of the ice is thicker than two meters, which would require more heat to melt it. If all the heat humans produced went into melting Arctic ice, there would be no increase in air temperature. To get both air temperature increase and Arctic ice melt, there has to be more heat than these numbers show. Obviously, the quantities are ridiculous for global warming melting Arctic ice. It's warm ocean water that melts Arctic ice, not carbon dioxide in the air. Ocean water is heated directly by sunlight and geothermal heat, not carbon dioxide. Geothermal heat is highly significant in heating the oceans, because it has thousands of years to accumulate, and it enters from below to heat the entire ocean. The Cause of Ice Ages
|